Write a Balanced Equation for the Reduction of 9 Fluorenone
2: Reduction of Organic Compounds (Experiment)
-
- Last updated
- Save as PDF
- Page ID
- 126792
Hydride based reducing agents LiAlH4 (lithium aluminum hydride) and NaBH4 (sodium borohydride) react with ketones and aldehydes to produce a 1° or 2° alcohol product. Both reagents were discovered by Schlesinger in the 1940s and are routinely used in organic synthesis. NaBH4 is a milder reducing agent than LiAlH4 and can be used in protic solvents, such as ethanol. Conversely LiAlH4 must always be used in aprotic solvents, such as tetrahydrofuran, and under extremely rigorous anhydrous (moisture free) conditions. LiAlH4 also requires a separate acidic work up step where reduction with NaBH4 does not. For more on these reagents see Eğe sections 14.4 and 21.3 B.Schlesinger, H.I.; Brown, H.C. et. al. J. Am. Chem. Soc. 1952, 75, 186.
Reaction Scheme
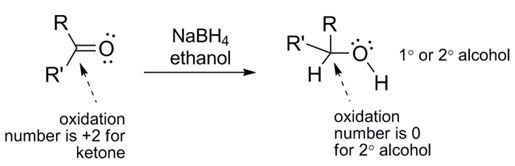
Note the stoichiometry of the reaction. Each molecule of NaBH4 can reduce up to 4 carbonyl groups since it delivers 4 hydrides per NaBH4 molecule. Keeping that in mind, try drawing the balanced equation for this reaction. Hint: one H on the alcohol comes from a hydride and the other comes from the acidic proton on CH3CH2OH (ethanol).
Techniques used
- Beilstein test
- melting point
- recrystallization
- extraction & wash
- drying agent
- gravity & vacuum filtration
- thin layer chromatography
- and infrared spectroscopy.
Objective
In part 1 you will reduce 9-fluorenone using the procedure described below. In part 2 you will reduce an unknown ketone also using the method below. Your objective is to determine if the ketone unknown can be reduced by NaBH4 to form an alcohol, to compare the two reactions (part 1 and part 2), and to determine the identity of the unknown ketone.
Procedure
Reagents
- 9-fluorenone (part 1)
- Unknown ketone (part 2)
- Ethanol
- NaBH4
In a dram vial, dissolve 0.1 g of 9-fluorenone (part 1) or unknown aromatic ketone (part 2) in 1 mL of 95% ethanol, and cool the solution in ice (most ketones will produce a fine suspension). Add to this solution or suspension 20 mg of sodium borohydride (a large excess). If you have a suspension the suspended ketone solid will dissolve. The reaction mixture should warm up. After 15 minutes, add 1 mL of water, heat the solution to the boiling point, dilute the hot solution with hot water (1-2 mL) to the point of saturation indicated by cloudiness.* Collect the crystalline precipitates generated upon cooling the mixture to room temperature using vacuum filtration. Recrystallize the reduction product. With the guidance of your GSI determine an appropriate solvent for recrystallization.
Note
Three of the unknown aromatic ketones should produce liquid products after reduction. If your unknown product is a liquid the mixture will not become cloudy upon addition of 2 mL of water. In this case you will perform a microscale extraction to isolate your product. Add 4-5 mL of diethyl ether to the mixture and mix to allow the product to migrate into the organic layer. Transfer the ether layer into another dram vial and wash with an equal volume of brine (saturated solution of NaCl). Pipet out the ether layer and dry it over anhydrous magnesium sulfate. Remove the magnesium sulfate by gravity filtration and evaporate the organic solvent by applying a stream of nitrogen gas.
Characterization
Determine the purity of the products in part 1 and 2, as well as the success of each reaction using TLC. Collect the Infrared spectrum of each product and the unknown ketone starting material. Characterize the starting materials and products of part 1 and 2 by m.p. Use the Beilstein test for halogens to rule out ketone product 1 (the only unknown with halogen) from table 2-1 below. Use Reaxsys or SDBS to search for literature IR spectra for the unknown ketone and its corresponding alcohol product for comparison to the spectra you obtain.
Write a Balanced Equation for the Reduction of 9 Fluorenone
Source: https://chem.libretexts.org/Ancillary_Materials/Laboratory_Experiments/Wet_Lab_Experiments/Organic_Chemistry_Labs/The_Synthesis_and_Characterization_of_Carbonyl_Compounds/2:_Reduction_of_Organic_Compounds_%28Experiment%29
0 Response to "Write a Balanced Equation for the Reduction of 9 Fluorenone"
Postar um comentário